Step by Step guide to using the Delphi Technique
What is the Delphi technique?
The Delphi Technique is a structured consensus-building approach that gathers expert opinion through a series of questionnaires.
By engaging a panel of knowledgeable stakeholders, such as clinicians, system leaders, or patient advocates, and facilitating structured rounds of feedback, the Delphi technique helps distil informed consensus around complex or evolving issues. Its anonymity reduces bias, encouraging honest responses and equal contribution.
This article outlines the key steps to plan, execute, and interpret a Delphi study in healthcare, where such evidence can influence real-world decision-making, shape policy, and ultimately improve outcomes.
When to use the Delphi technique?
The Delphi technique is most often employed in situations where there is no clear-cut evidence on which to rely for answers. This may include forecasting, estimating future risks, generating new evidence, and policy making.
Within healthcare, medical teams often use the Delphi technique to define and prioritize unmet medical needs (e.g., identifying patients most likely to benefit from a new therapy), refine clinical practice (e.g., establishing diagnostic criteria or therapeutic algorithms), and contribute to guideline development. For this reason, other professionals working in market access or policy roles will also find this and effective approach..
Using the Delphi technique: step-by-step guide
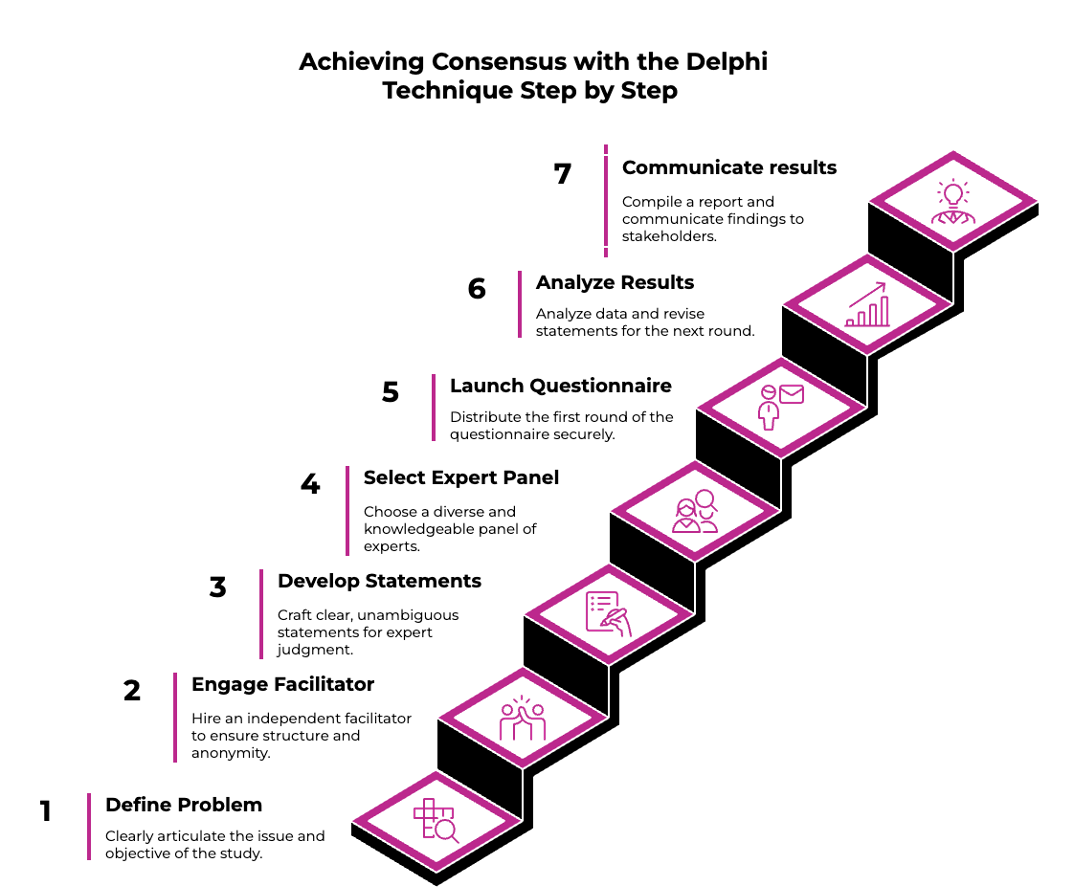
Step One – Define the problem and aim of the project
Begin by clearly articulating the issue you aim to address and the objective of the study. This should be informed by a thorough review of current evidence, guidelines, and stakeholder needs.
Step Two – Employ a Delphi facilitator
To deliver a Delphi consensus appropriately, engaging an independent facilitator is necessary to ensure correct structure, anonymity of responses and minimisation of any bias. You can read our article on How To Select A Consensus Company and download our check-list to help you evaluate a potential partner.
Step Three – Develop statements
Statements form the core of a Delphi study. Each statement should present a single, unambiguous concept that invites expert judgment, typically rated on a Likert scale. Ensure statements comprehensively reflect the issue from different perspectives (clinical, operational, patient-centric), while avoiding double-barrelled or overly complex phrasing. Well-crafted statements lead to stronger consensus and clearer interpretation.
Step Four – Engage a relevant expert panel
Select a diverse and knowledgeable panel tailored to the topic. This may include clinical experts, researchers, policy advisors, patient representatives, or system stakeholders. While there is no fixed number of participants, a panel size of at least 8–20 experts is often sufficient. In broader or more variable situations, a larger sample may enhance credibility and generalizability. Panel diversity across geography, discipline, and setting strengthens the value of consensus.
Step Five – Launch the first round of questionnaire to the panel
Distribute the first round of the questionnaire via a secure survey platform. Ensure clarity in instructions and consistency in response scales. Anonymity should be maintained to encourage honest feedback and avoid influence from seniority or status.
Step Six – Analyse results & launch second round of questionnaire
After each round, analyze the data quantitatively (e.g., % agreement) and qualitatively (e.g., recurring themes or outlier rationale). A commonly accepted threshold for consensus is ≥75% agreement, though this should be pre-specified. Statements that do not meet the threshold can be revised and redistributed for another round, allowing participants to consider the group feedback and adjust their responses. This iterative process typically leads to convergence on key issues.
Step Seven – Provide recommendations and support action
Once consensus is reached, compile a summary report highlighting key outcomes, themes, and opportunities for change. Submitting the study for peer-reviewed publication is strongly recommended to add credibility and support future citation. This also lays the foundation for broader communication across relevant audiences and channels. Communication activities can then be launched across different audiences and mediums, as outlined in our article on How to Maximise Impact of a Delphi Study.
Examples of the Delphi technique in use
View the case studies below to see how Triducive have employed the Delphi approach to support clients and drive positive change.
Triducive create groundswell of credible opinion to positively influence EU antibiotic policy
Triducive develop aligned recommendations for managing hyperkalaemia across the cardiorenal spectrum
Triducive help a leader in respiratory medicine to improve referral pathways in the United Kingdom
Wondering if you should embark on a Delphi study to support your plans?
Take our 2-minute test to find out.
Ready to plan your Delphi study?
Learn more about the expert team behind Triducive that generates consensus-led evidence that gets published and supports change, collaborating with medical affairs and other teams in life sciences all around the world. We have been delivering 130+ Delphi consensus to date, helping to change practice and guidelines, improve pathways, and support payers’ decisions.