Designing Global Delphi Panels to Deliver Actionable Insights
Pharmaceutical companies, medical affairs teams, and guideline developers frequently require global or multi-regional consensus to:
- Inform global clinical guidelines and align recommendations across markets
- Support regulatory and payer engagement with robust international perspectives
- Enable effective product launches, aligned messaging, and pertinent educational initiatives
- Identify implementation barriers and regional differences affecting adoption
- Drive strategic alignment across internal stakeholders and expert networks
Achieving consensus across multiple countries is complex. Variations in healthcare systems, cultural norms, and operational logistics introduce challenges that do not exist in single-country studies. Global Delphi panels, which use structured, iterative methods to elicit expert agreement, provide a solution, but their design and interpretation require careful consideration.
The Influence of Healthcare Systems
Expert responses in a global Delphi panel can be shaped by local healthcare environments, including:
- Reimbursement and payer structures
- National or regional treatment guidelines
- Access to diagnostics and therapies
- Regulatory frameworks
- Care pathway infrastructure
- Cultural norms around risk and clinical decision-making
For example, an oncologist in a publicly funded European system may interpret a treatment algorithm differently than a US clinician operating under private insurance. In emerging markets, feasibility and resource constraints may weigh more heavily than trial endpoints.
Apparent disagreement in a global Delphi panel may therefore reflect systemic differences rather than scientific dissent. Global consensus is rarely about uniform agreement; it is about mapping where alignment exists and identifying regional adaptation needs.
Cultural Considerations
Cultural context can influence Delphi responses. Factors such as willingness to disagree, interpretation of rating scales, comfort with ambiguity, and response extremity can vary across regions.
- Some cultures prioritise harmony, reducing overt dissent.
- Others encourage assertive disagreement.
- Hierarchical cultures may suppress deviation from perceived authority, even in anonymous surveys.
To mitigate these biases, global Delphi panels should include:
- Neutral and unambiguous phrasing of statements
- Rigorous translation ensuring conceptual equivalence
- Clear explanation of rating scales
- Strong anonymity protocols
- Structured, balanced feedback between rounds
Cultural literacy is not optional, it is a methodological necessity in multi-regional studies.
Operational Considerations
Designing and conducting a global Delphi panel requires careful operational planning, including:
Panel composition: A balanced representation must be maintained across regions, practice settings, healthcare system types, and seniority. Over-representation of one region can skew global agreement and impact generalisability in interpreting results.
Language and translation: Clinical terminology must maintain conceptual integrity across languages. Misinterpretation can introduce hidden bias.
Regulatory and policy context: Experts must understand whether to respond purely clinically or within local constraints, as different regions have varying evidence thresholds and implementation frameworks.
Engagement logistics: Time zones, asynchronous feedback, and response windows must be managed to maintain participation and minimise panel fatigue.
Interpreting Global Consensus
A common misconception is that global consensus equates to a single aggregate agreement percentage. In reality, regional variation can provide more meaningful insights:
- 80% global agreement may mask strong consensus in Europe but moderate divergence in Asia.
- 65% agreement may reflect constraints in one region despite near-unanimity elsewhere.
A well-designed global Delphi panel analyses both aggregate and regional results. Key questions include:
- Where is alignment strongest?
- Where does divergence occur, and why?
- Are differences driven by clinical interpretation, system constraints, or cultural factors?
- How should findings inform regional messaging, education, or policy engagement?
Consensus should illuminate differences, not obscure them.
Designing for Strategic Impact
To generate actionable insights, a global Delphi panel must be designed around its intended decision:
- Support global guideline development
- Inform pre-launch strategy
- Strengthen payer engagement
- Align messaging across markets
This approach ensures appropriate geography, statement framing, pre-planned regional analysis, and outputs tailored to strategic use. Without this alignment, even a methodologically rigorous panel may yield academically interesting but practically underutilised findings.
Divergence as Insight
Disagreement is not failure. Regional divergence can reveal:
- Access barriers
- Infrastructure gaps
- Educational needs
- Policy constraints
- Variability in guideline adoption
For medical, commercial, and policy teams, understanding divergence is often more actionable than observing uniform agreement. The aim of a global Delphi panel is not to eliminate difference, but to clarify it.
Conclusion
While healthcare innovation is increasingly global, implementation remains local. A global Delphi panel offers a structured method to:
- Capture international expert alignment
- Identify regional nuance
- Anticipate implementation challenges
- Inform differentiated market strategies
When designed with methodological rigor, cultural sensitivity, and strategic intent, global consensus becomes more than an academic exercise. It is a tool for clarifying complexity and transforming diversity into actionable insight.
Discover how Triducive can help
Triducive is an expert medical communications agency that generates consensus-led evidence, which is published and supports change in healthcare.
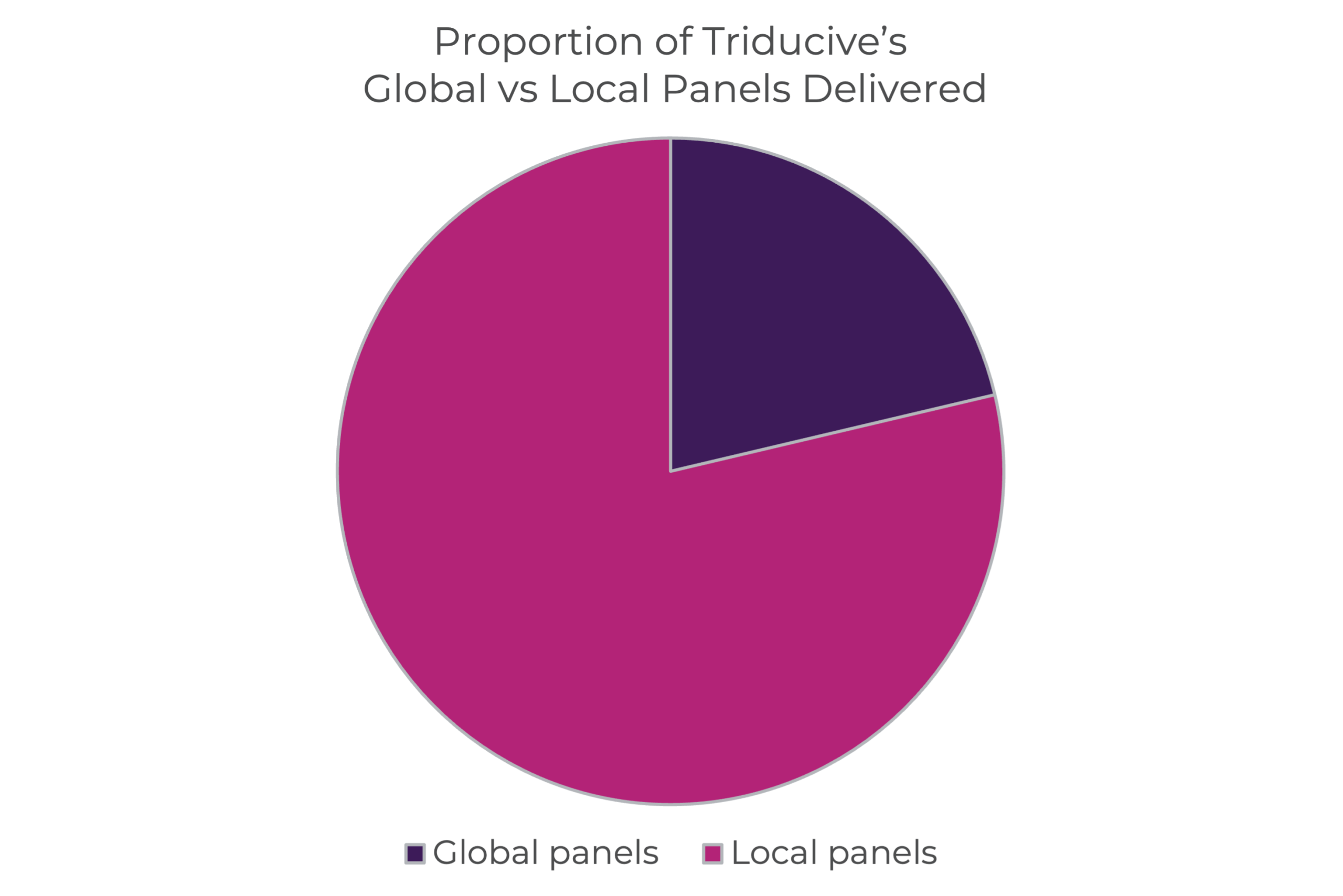
Triducive has strong experience in facilitating both global and local Delphi panels. Around 30% of the studies conducted by Triducive are global.
Our team of healthcare experts and scientific writers is ready to provide guidance and support at any stage of the evidence generation process. Contact us for more information.